OXYCODONE/NALOXONE1,2

12-hourly dosing
ARX-Oxycodone/Naloxone oxycodone hydrochloride/ naloxone hydrochloride
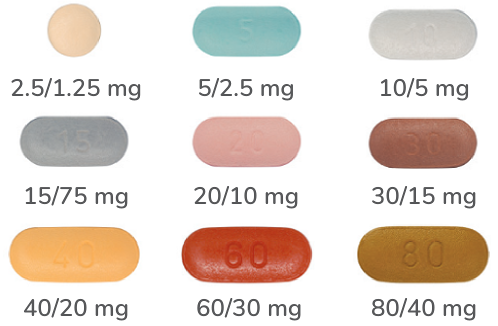
OXYCODONE3
12-hourly dosing
MORPHINE4
MS Contin® tablets
modified release
morphine sulfate
12-hourly dosing

BUPRENORPHINE5,6

transdermal buprenorphine
matrix patch
7-day dosing

BUPREDERMAL® patch
transdermal buprenorphine
matrix patch
7-day dosing

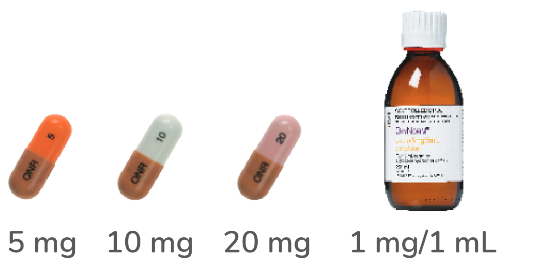
OXYCODONE7,8

capsules & liquid
immediate release
oxycodone hydrochloride
4- to 6-hourly dosing
Capsules must not be opened, chewed or crushed

injection or infusion
oxycodone
hydrochloride
injection or infusion

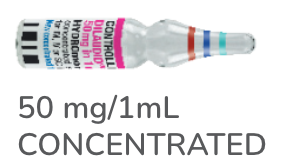
HYDROMORPHONE9,10
DILAUDID®/ DILAUDID®-HP injection
hydromorphone hydrochloride
injection or infusion
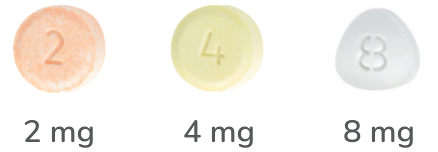
HYDROMORPHONE9,10
DILAUDID® tablets
immediate release hydromorphone hydrochloride
4-hourly dosing
Mundipharma is not promoting the use of strong opioids in chronic non-cancer pain.
Please review Product Information and State and Federal regulations before prescribing.
For full TARGIN® tablets prescribing information, please click here.
For full ARX-Oxycodone/Naloxone tablets prescribing information, please click here.
For full OxyContin® tablets prescribing information, please click here.
For full MS Contin® tablets prescribing information, please click here.
For full NORSPAN® patch prescribing information, please click here.
For full Bupredermal® patch, prescribing information, please click here.
For full OxyNorm® capsules and liquid prescribing information, please click here.
For full OxyNorm® injection prescribing information, please click here.
For full DILAUDID® tablets prescribing information, please click here.
For full DILAUDID®-HP Injection prescribing information, please click here.
Attention should be given as the use of opioids has a risk of addiction, misuse and abuse. Therefore, appropriate assessment and monitoring is required at initiation, maintenance and tapering of opioid therapy.
Limitations of use: Because of the risks associated with the use of opioids, TARGIN® Modified Release Tablets, ARX oxycodone/naloxone tablets OxyContin® modified release tablets, MS CONTIN® modified release tablets, NORSPAN® patch, BUPREDERMAL® patch, OxyNorm® capsules and liquid, OxyNorm® solution for injection or infusion, DILAUDID® tablets, DILAUDID®/DILAUDID®-HP INJECTION should only be used in patients for whom other treatment options, including non-opioid analgesics, are ineffective, not tolerated or otherwise inadequate to provide appropriate management of pain.
There are risks of hazardous and harmful use which can lead to overdose and death; life threatening respiratory depression. Concomitant use of benzodiazepines, gabapentinoids, antihistamines, tricyclic antidepressants, anti psychotics, cannabis or other central nervous system (CNS) depressants, including alcohol may result in profound sedation, respiratory depression, coma, and death. For full details, please see section 4.4 Special Warnings and Precautions for Use in full Product Information.
PBS Information: TARGIN® tablets, ARX oxycodone/naloxone tablets, NORSPAN® patch, Bupredermal® patch, OxyContin® tablets, MS Contin® tablets. Authority required (STREAMLINED 10755, 10748, 10752). Chronic severe pain. Refer to PBS schedule for full authority information, including increased maximum quantities and/or repeats.
PBS Information: MS Contin® tablets (200mg). Authority required. Chronic severe disabling pain. Refer to PBS Schedule for full Authority Required information.
PBS Information: OxyNorm® capsules, OxyNorm® liquid, DILAUDID® tablets, Restricted benefit. Severe pain. Refer to PBS schedule for full restricted benefit information. Authority required for increased maximum quantities and/or repeats. Refer to PBS Schedule for full Authority Required information.
PBS Information: OxyNorm® injection and DILAUDID®-HP 50mg in 1mL are not listed on the PBS.
Adverse events should be reported. Reporting forms and information can be found at https://aems.tga.gov.au/. Adverse events can also be reported to Mundipharma at drugsafety@mundipharma.com.au.
References:
1. TARGIN® Modified Release Tablets Product Information, June 2023.
2. ARX-Oxycodone/Naloxone Modified Release Tablets, October 2024.
3. OxyContin® Modified Release Tablets Product Information, November 2024.
4. MS CONTIN® modified release tablets Product Information, July 2024
5. NORSPAN® PATCH Product Information, June 2025.
6. BUPREDERMAL® PATCH Product Information, June 2025.
7. OxyNorm® Capsules and Liquid Product Information, May 2025.
8. OxyNorm Injection® Product Information, November 2024.
9. DILAUDID® TABLETS Product Information, May 2024.
10. DILAUDID®/DILAUDID®-HP INJECTION Product Information, May 2024.
® TARGIN, ARX-Oxycodone/Naloxone, NORSPAN, BUPREDERMAL, OXYCONTIN, OXYNORM, MS CONTIN and DILAUDID are registered trade marks of Mundipharma. Mundipharma Pty Limited ABN 87 081 322 509. Sydney, NSW 2000. Tel: 1800 188 009. FD23487
AU-TARG-2500008 Prepared: July 2025













